A new nanocatalyst that recycles major greenhouse gasses, such as carbon dioxide (CO2) and methane (CH4), into highly value-added hydrogen (H2) gas has been developed. This catalyst is expected to greatly contribute to the development of various waste-to-energy conversion technologies, as it has more than twice the conversion efficiency from CH4 to H2, compared to the conventional electrode catalysts.
A research team, led by Professor Gun-Tae Kim in the School of Energy and Chemical Engineering at UNIST has developed a novel method to enhance the performance and stability of catalysts, used in the reaction (i.e., dry reforming of methane, DRM) that produces H2 and carbon monoxide (CO) from well-known greenhouse gasses, such as CO2 and CH4.
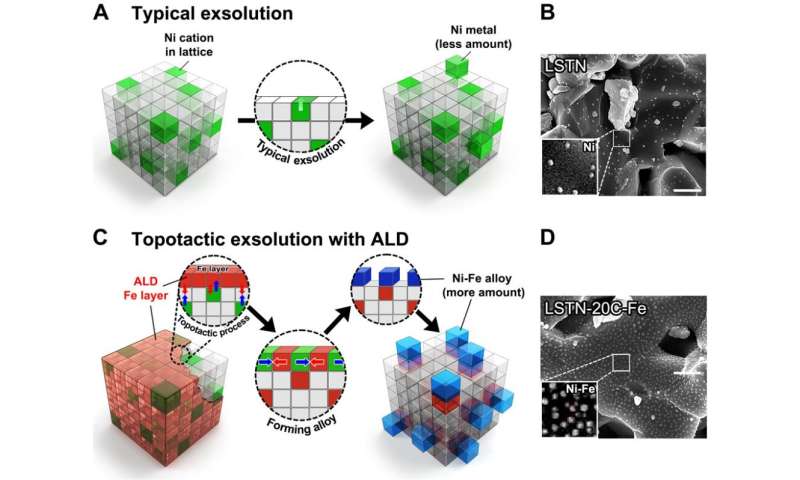
The conventional catalysts used for the dry reforming of methane (DRM) are nickel (Ni)-based metal complexes. Over time, however, the performance of catalysts degrade, so does the catalyst lifetime. This is because carbon accumulates on the surface of the catalysts, as the catalysts clump together or their reaction is repeated at a higher temperature.
"The uniform and quantitatively controlled layer of iron (Fe) via atomic layer deposition (ALD) facilitates the topotactic exsolution, increasing finely dispersed nanoparticles," says Sangwook Joo (Combined MS/Ph.D. in the School of Energy and Chemical Engineering, UNIST), the first author of the study.
The research team also confirmed that exsolution is promoted even with a very small amount of ALD-deposited Fe oxide (Fe2O3). "Notably, at 20 cycles of Fe oxide deposition via ALD, the particle population reaches over 400 particles (Ni-Fe alloys)," says Arim Seong of the School of Energy and Chemical Engineering, UNIST, the first co-author of the study. "As these particles are composed of Ni and Fe, they also exhibited high catalytic activity."
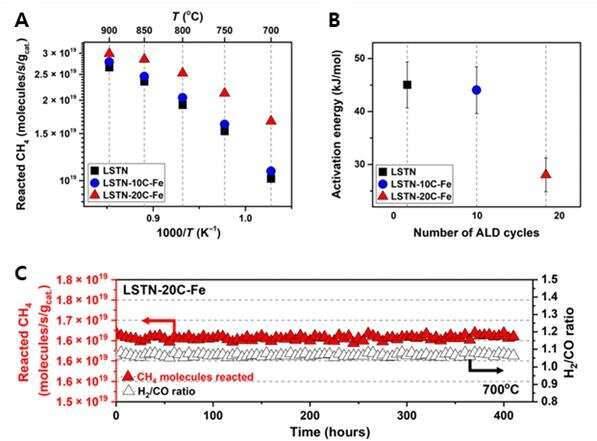
The new catalyst exhibited high catalytic activity for the DRM process with no observable degradation in performance for more than 410 hours of continuous operation. Their results also showed a high methane conversion (over 70%) at 700 degrees C. "This is more than twice the power conversion efficiency that of the conventional electrode catalysts," noted Professor Kim. "Overall, the abundant alloy nanocatalysts via ALD mark an important step forward in the evolution of exsolution and its application to the field of energy utilization."
Explore further
Citation: New catalyst turns greenhouse gases into hydrogen gas (2020, October 26) retrieved 26 October 2020 from https://ift.tt/31EPbKo
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.
"gas" - Google News
October 26, 2020 at 07:27PM
https://ift.tt/31EPbKo
New catalyst turns greenhouse gases into hydrogen gas - Phys.org
"gas" - Google News
https://ift.tt/2LxAFvS
https://ift.tt/3fcD5NP
Bagikan Berita Ini














0 Response to "New catalyst turns greenhouse gases into hydrogen gas - Phys.org"
Post a Comment